Site Focus: How the MCTM solves specific systemic issues in recruitment for sites
by Brandon Li, Co-Founder
In our blog post, Introducing a Modern Clinical Trial Marketplace, a new approach to patient recruitment, we introduced the concept of a Modern Clinical Trial Marketplace as a way to solve the biggest challenges in patient recruitment today. And in the follow up, Patient focus: How the MCTM solves specific systemic issues in recruitment for patients, we focused on how the Modern Clinical Trial Marketplace solves recruitment challenges for patients.
In this blog, we focus on how the Modern Clinical Trial Marketplace solves recruitment challenges for sites.
Quick recap:
- The Modern Clinical Trial Marketplace (MCTM) aims to address systemic issues in recruitment by creating a marketplace where all 3 core stakeholders - patients, sites and sponsors - can engage with each other seamlessly.
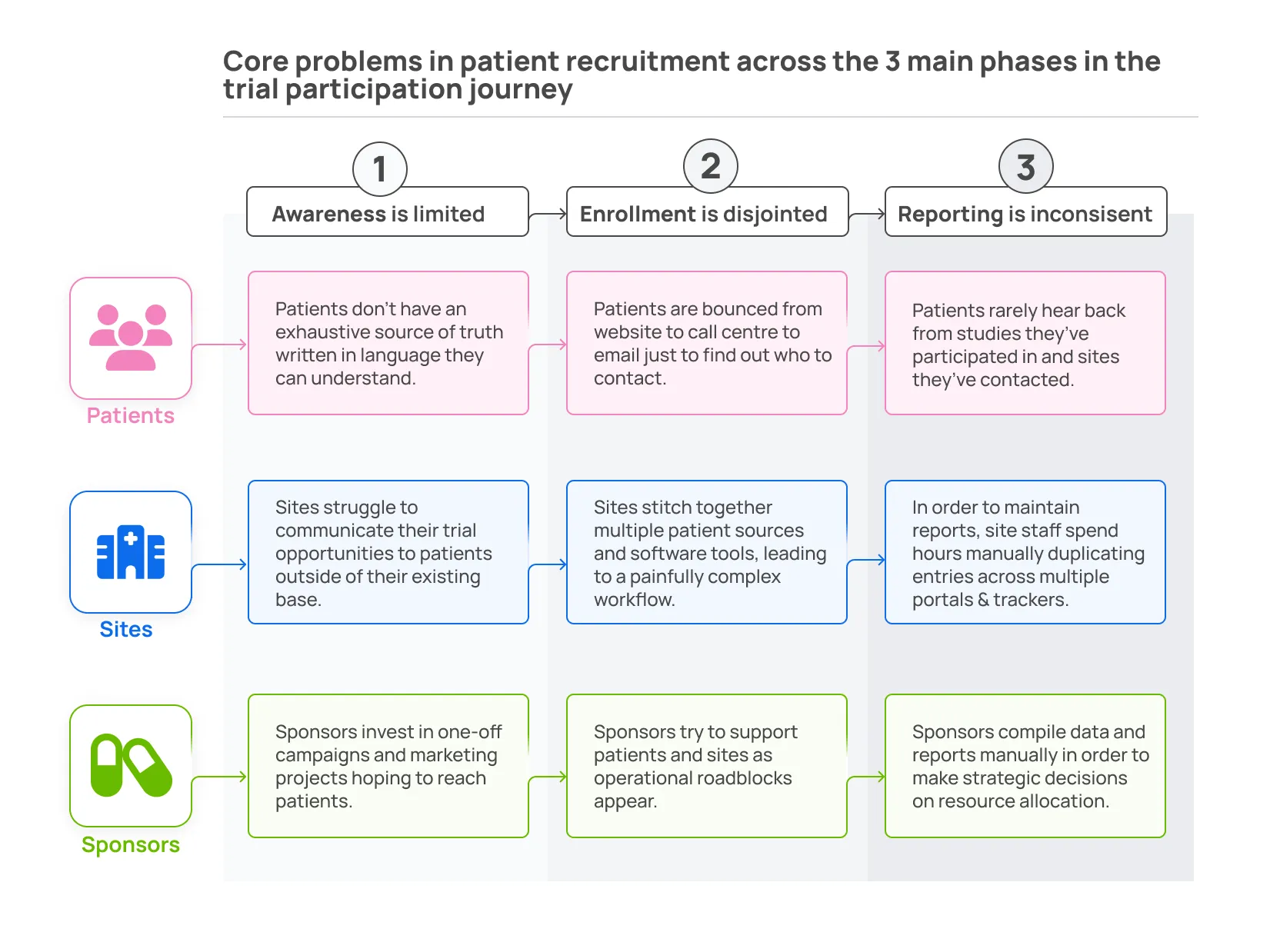
- Let’s break down how an MCTM can solve fundamental problems for patients, sites, and sponsors throughout the trial journey. The summary table below breaks down the core problems in patient recruitment across the three main phases in the trial participation journey: awareness, enrollment, and reporting. We examine these stages from the perspective of our three core stakeholders: patients, sites, and sponsors.
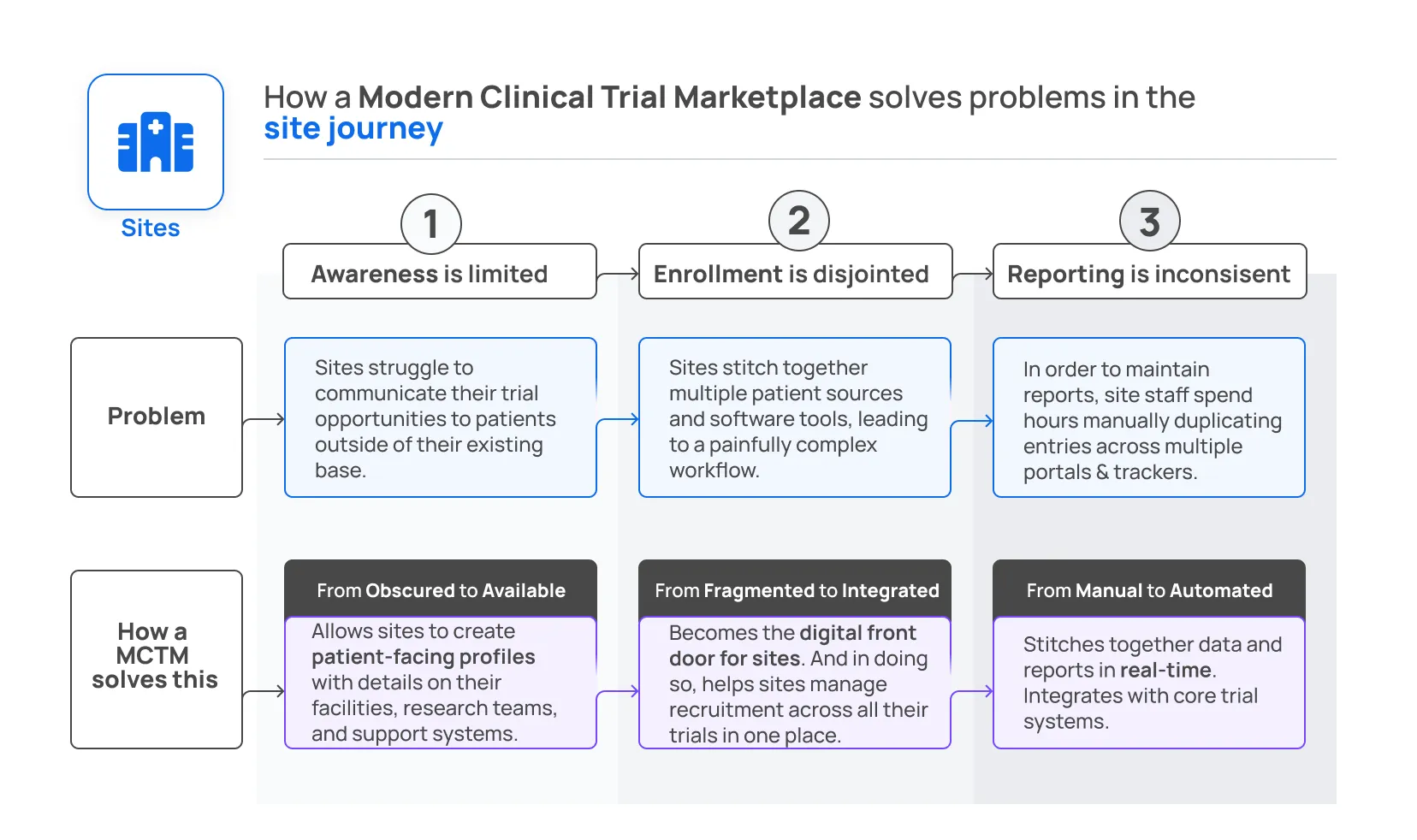
Part 2: Solving the site’s problems
“We use several different recruitment platforms - sometimes sponsors require we use a certain platform, sometimes they just give us a set budget and we need to make the recruitment decisions ourselves…it’s definitely frustrating to amalgamate all these resources. Our recruitment coordinator basically spends her days switching between everything and copying notes across platforms.”
- Industry clinical trial site
Eligible patients live and work around trial sites, but they are obscured from these sites because no large-scale database exists. The lack of a centralized database means that sites are primarily limited to patients who already receive care at their clinic.
In response, sites use a suite of fragmented recruitment tools. These tools don’t integrate, require oversight and training, and often don’t do a great job recruiting new prospects.
Furthermore, the lack of integration requires hours of manual data entry and duplication across trials. This time could be spent giving care or doing community outreach. Researchers are busy and overwhelmed so the Marketplace needs to lighten, not burden their workload. They should choose the platform instead of having the platform forced upon them.
How a Modern Clinical Trial Marketplace creates an available, integrated, automated recruitment platform for sites.
A successful Marketplace will be attractive because all the patients using the platform will be available to sites. Sites can update their profile with details about their facilities, research teams, and support systems to make themselves more attractive to patients as well.
But it’s not enough to connect patients and researchers. The Marketplace should also reduce the burden on site staff because it is integrated instead of fragmented. Sites can access the Marketplace, understand it quickly, and get started without any training, IT integrations, or sales calls. This industry needs less friction.
Finally, a Modern Clinical Trials Marketplace should allow sites to create profiles to capture patient interest, track patient progress through enrollment, and automatically send necessary info to sponsors without additional hours spent on manual data entry.
How this looks in practice on Power
- Frictionless onboarding: All sites can onboard in minutes without any support from IT, and start matching with patients.
- Pre-screened patients: Sites receive pre-screened patients matched to the protocols of each study, and can adapt the pre-screener in real time.
- Comprehensive patient profiles: Sites have full visibility into all the information the patient provided when they apply, including all demographic data.
- Patient Registry: Sites can see patients who have applied to similar trials and can actively reach out to qualified participants.
- Real time reporting: Data flows in real time and sites can easily share recruitment progress, and demographic information.
In our next blog post, we’ll deep dive into how a Modern Clinical Trial Marketplace addresses the current systemic issues we see across the patient recruitment journey - Awareness, Enrollment and Reporting - for sponsors.
Have you already read the patient focus blog? Check it out here.