Clinical Trial Phases Explained: The Ultimate Guide
Introduction: What are clinical trials?
Clinical trials are research studies designed to test new drugs, already approved drugs, devices, or other forms of treatments. They are the main way that researchers test if a treatment, like a new drug, medical device or surgical intervention is safe and effective in people. Clinical trials help answer several questions regarding the safety and efficacy of a drug or treatment:
- Is the treatment effective for managing and/or treating the condition?
- What is the optimal dose for treating the condition while minimizing side effects?
- Is it more effective than existing treatments?
- How well is the treatment tolerated by patients?
- What is the frequency and severity of side effects, if any?
- Does it work differently in different groups or types of people?
Clinical trial designs need to be reviewed and approved by the FDA before the trial can be started. The trial is then conducted according to a comprehensive protocol outlining all aspects of the study. In general, a drug passes through different clinical trial phases, which are separate studies, but which build upon the insights gained in earlier phases by asking different questions and gathering more data.
The end goal of clinical trials is to demonstrate the drug’s efficacy with robust data and ensure its safety for the general population. We will go through the whole process, in order to paint the whole picture of a new drug's journey through the clinical research process, from conception to approval for use in patients. Here is what you will find in this article:
1. The Basics: What is clinical research?
- Brief overview of the clinical research process
- Need-to-knows about clinical trials and clinical phases
2. The Details: What are the 4 phases of clinical trials?
- Phase I Clinical Trials (Dose-escalation studies)
- Phase II Clinical Trials (Dose-finding studies)
- Phase III Clinical Trials (Pivotal studies)
- Phase IV Clinical Trials (Post-marketing surveillance studies)
3. Conclusion
The Basics: What is clinical research?
Before we dive deeper into descriptions of the different clinical trial phases, we will first explore the clinical research process in general. Clinical trials are one part, or stage, of clinical research.
Clinical research refers to the comprehensive, multi-stage study of drugs to evaluate their effectiveness and safety in healthcare. Clinical research can also be done for medical devices, which go through distinct stages. When a new prospective drug is conceived, it is first subjected to certain tests in the laboratory, which are also called preclinical studies. If preclinical study results indicate it has a high potential of being successful for a given condition, the drug can be submitted for approval as an IND, and clinical studies can be designed.
A drug must then be investigated through clinical trials before it can be approved for marketing and use in humans. The infographic below provides a visual representation of the stages of clinical research, which are described in the following section.
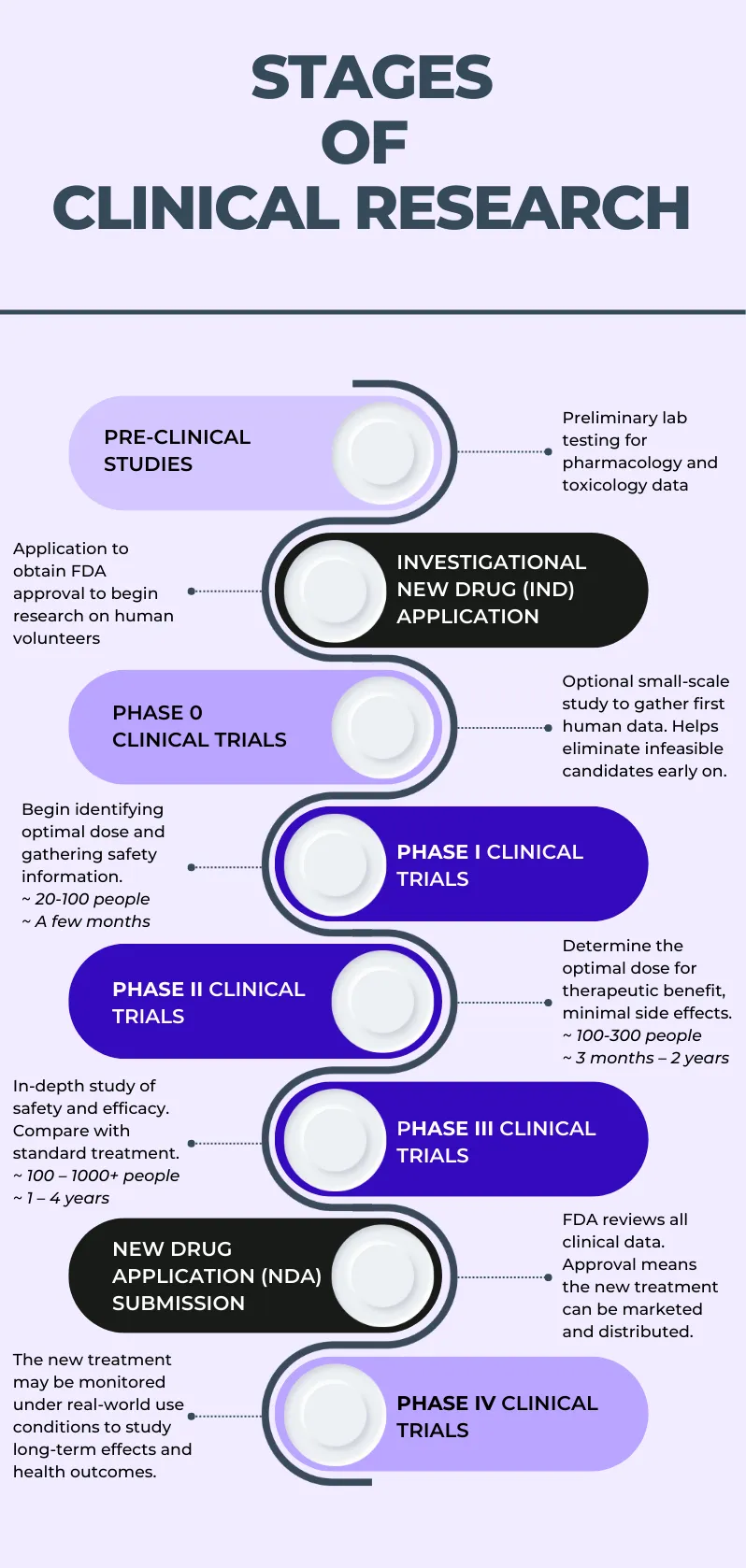
Figure 1. Schematic illustration of the stages involved in the clinical research process
Brief overview of the clinical research process
Pre-clinical (laboratory) studies
Pre-clinical studies, or laboratory studies, include cell and animal studies performed in the laboratory, and provide useful preliminary information. However, real-world use conditions are far different from laboratory conditions, so if preclinical studies indicate that the new drug is likely to be safe and effective for its intended purpose, the drug may progress to clinical trials so investigators can study its use in human participants.
Investigational New Drug (IND) application
Before clinical trials can begin, an investigational new drug (IND) application must be filed with the FDA. A drug must be registered as an IND to permit research to be conducted on human volunteers. The IND application must include complete information concerning three general areas:
- Animal toxicology and pharmacology studies
- Manufacturing information
- Investigator information and clinical protocols
For further detail and instructions for sponsors, please see the FDA IND page.
The FDA will notify the applicant regarding the date the submission is received through an IND acknowledgement letter. The IND will then enter into effect within 30 days of its reception, unless it is subjected to “Clinical Hold,” in which case all investigations are stopped and a process can be started to resolve the issues identified. No trials may begin, and ongoing studies must be stopped, until the applicant is notified that the clinical hold has been lifted.
Note that there are certain cases in which investigations can be conducted without needing to submit an IND. Details about which cases are included among these exemptions can be found here.
IND meaning:
IND stands for Investigational New Drug, which describes any prospective drug going through the various stages of clinical research, before it is approved for marketing through an NDA.
Phase 0 clinical trials
Phase 0 clinical trials, also known as "exploratory IND studies," were introduced by the FDA in 2006 but are not always conducted before Phase I clinical trials. They are small-scale studies designed to gather preliminary insights into the drug’s basic safety profile and metabolism in humans, essentially aimed at eliminating infeasible drug candidates before significantly more resources would be invested in a Phase I trial that ends up leading to the same conclusion. In Phase 0 studies, human participants receive sub-therapeutic doses of the new drug to allow researchers to study how the drug reacts with the human body, including how it is absorbed and metabolized and whether there are any immediate side effects. If a Phase 0 clinical trial is not conducted for a given drug, the first clinical trial would be a Phase I clinical trial.
Phase I-III clinical trials
Phase I, Phase II, and Phase III clinical trials are consecutive yet separate studies which involve progressively refined hypotheses and research objectives aimed at collecting increasing amounts of data about the new drug’s optimal dosage and route of administration, its tolerability and safety profile, and its therapeutic effect or potential. All of these phases involve human volunteer participants, typically both healthy volunteers as well as patients with the condition the drug aims to treat, with the later phases generally involving an increasing number of participants and longer timeframes. Phase I through III clinical trials represent the bulk of data collection in clinical research - details about each phase are provided further on in this article.
New Drug Application (NDA) submission: FDA approval
After successful completion of a phase III clinical trial, if the IND continues to look promising as a treatment, a New Drug Application (NDA) is filed with the FDA, which acts as a formal request for permission to market the drug. The NDA contains any and all information that is relevant to the drug, including data from preclinical testing and from all clinical trial phases, its metabolism and pharmacodynamics, tolerability and safety profile, and manufacturing and packaging.
The NDA should allow the FDA to clearly determine whether the drug is safe and effective for its intended use, pure and manufactured according to strict standards, and likely to provide benefit to patients. The NDA is submitted via an FDA Form 356h. If the NDA is approved, then the drug can officially be marketed and prescribed or given to patients in the real-world setting. For further information, see the NDA resource on the FDA’s website.
Phase IV clinical trials (post-marketing surveillance studies)
When the drug has been approved by the FDA and is being given to patients, it may be placed under continued surveillance to monitor its long-term performance (as assessed by various different metrics) in a larger population group. These “post-marketing surveillance” studies are important for answering some important questions regarding the effectiveness and safety of the drug or treatment.
Need-to-knows about clinical trials and clinical trial phases
Before further explaining the four main phases of clinical trials, we wanted to provide some information that might be helpful if you’re thinking about participating in clinical trials.
- Participation in clinical research is voluntary. Taking part in a clinical trial is a personal decision, and all clinical trial participation is voluntary. If you are considering participating in a clinical trial, it’s important to gather information about the trial, ask questions about anything you would like to know more about, clarify anything you might not fully understand, and consult with your doctor and family members, so you can make an informed decision about whether or not to participate in a clinical trial.
- Participants may withdraw at any point in the study. Any participant may withdraw from the study voluntarily at any moment, including while the trial is in progress. The research team is always available to help you navigate any questions that might arise.
- Participants enroll in just one trial phase. Each phase is a separate study and involves separate enrollment and consenting processes. Patients do not "go through" clinical trial phases, but the study drugs/treatments do.
- Participant safety is of utmost importance. Safety is given utmost priority throughout every phase and stage of the clinical research process. External, third-party reviewers and regulatory agencies are responsible for approving all studies, with particular attention given to the safe and ethical treatment of patients.
- All clinical trials phases are necessary. While patients do not need to join all phases of a trial, the drug/treatment being tested must pass through all phases to be approved for public use.
The details: What are the 4 phases of clinical trials?
Clinical trials represent the stage of the clinical research process that involves human subjects. The 4 phases of clinical trials are Phase I clinical trials, Phase II clinical trials, Phase III clinical trials, and Phase IV clinical trials. Some aspects of the different phases may overlap or blur; for example, safety information is collected throughout all phases. In this section, we will take a closer look at each phase.
Phase I Clinical Trials (Dose-Escalation Studies)
Phase I clinical trials are designed to study the effects of the new drug or treatment on human subjects, typically for the first time. They may be called first-in-human (FIH) studies. Around 20-100 volunteers (typically healthy volunteers, although people with the disease can also be asked to participate) are gathered to test the safety of the new drug/treatment, and to establish the best way to administer the drug as well as the maximum tolerable dosage (MTD) – the highest dose that is unlikely to cause any serious side effects. Participants initially receive a small dose of the drug. If no side effects are apparent, the dosage is increased. This process continues until the ideal dosage is determined. For this reason, Phase 1 clinical trials may also be called “dose-escalation studies.”
Phase I clinical trials typically last a few months, and approximately 70% of study drugs proceed to Phase II clinical trials.
Phase II Clinical Trials (Dose-finding studies)
Phase II clinical trials involve testing the new drug in a larger group of volunteer patients, i.e., 100-300 people with the condition that the drug is designed to manage or treat. A primary aim of phase II trials is to identify the most successful dose (MSD) – the optimal dose for conferring therapeutic benefit to patients without serious side effects. Thus, phase 2 clinical trials may sometimes be called “dose-finding studies.” Further information about the safety and effectiveness of the drug is gathered by administering theoretically optimal dosages to a larger group of people over a longer period of time.
Phase II clinical trials can last from a few months up to 2 years, and only approximately 33% of study drugs move on to the next phase.
Phase III Clinical Trials (Pivotal Studies)
Phase III clinical trials are the most extensive and thorough clinical trial phase and involve the greatest number of participants, usually between one hundred to a few thousand. Phase III clinical trials are usually conducted at multiple sites, potentially across different countries, in order to enroll a wider population and gather data from a larger sample. Phase III trials tend to last from 1 to 4 years, although there is no standard timeline.
If a drug is found to be promising based on Phase III trial results, a New Drug Application (NDA) is filed with the FDA. The NDA is essentially an authorization for marketing, so if the NDA is approved, the drug can be marketed and made available as a treatment for the general population. This is why Phase III studies are also known as "pivotal” or “pre-marketing” studies.
Phase III clinical trials can adopt various different study designs, such as randomized controlled trials (RCTs), uncontrolled trials, factorial designs, historical controls, and sequential group designs. In RCTs, which are considered the ‘gold standard’ of clinical research studies, participants are divided into randomly-assigned groups; the control group wherein participants receive a placebo or the standard treatment, and the study group wherein participants receive the new drug. This allows for a direct comparison of the new drug against either a placebo or the current standard treatment, in order to determine whether it represents a substantial improvement upon existing treatment options.
Since phase 3 trials focus on monitoring side effects and adverse reactions in a wider population and over a longer timeframe, they help gather significantly more safety data. Phase III clinical trials tend to offer the most potential therapeutic benefit to the participants as the optimal dose has now been established and the study lasts longer. Nonetheless, providing therapeutic benefit is still usually not one of the main objectives of this type of clinical trial
Approximately 25-30% of drugs make it past phase III clinical trials and IND approval.
Phase IV Clinical Trials (Post-Marketing Surveillance Studies)
After NDA approval, the new drug can be made available as a treatment option for the general public. However, the clinical research usually does not stop there. Also known as post-marketing surveillance studies, phase IV clinical trials involve monitoring the safety and performance of the drug over the long-term, and most importantly, in real-world use conditions. Real-world use differs from the conditions of phase I-III clinical trials, since the people taking the drug are not participating actively in any study, and there are no longer any factors which are controlled (and thus potentially influenced) by the researchers. All studies conducted after final FDA approval of a drug are included in Phase IV clinical trials, which represent a mix between clinical research and clinical practice.
Phase IV clinical trials can be designed to assess the performance of the new drug using one or more different metrics, including its effectiveness in treating the condition (treatment outcomes), side effects of long-term use, incidence of adverse events, and economic factors such as healthcare costs to patients and/or insurance companies. Results of phase IV studies can be used in decisions such as restricting its use to certain conditions or even removing it from the market entirely. Sometimes, NDA approval can be conditional upon conducting specific phase IV trials to begin to study its real-world use right away. It is also relevant to note that the drug developer is rarely the initiator of phase IV studies, which are more commonly initiated by other actors in the healthcare industry or by government regulatory agencies.
The number of subjects providing data for phase IV trials depends on the number of people using the drug or treatment. Similarly, the length of Phase IV clinical trials is entirely dependent on the information collected and any potential concerns detected.
Conclusion
Information is power, and the more you understand about your condition, your trial, and the clinical research process in general, the more empowered you can be to play an active role in your healthcare decisions and the management or treatment of your condition. Hopefully this article has helped you understand the different phases of clinical trials and why they are all important.
Clinical trials are becoming increasingly "patient-centric" - focused on you, the participant, and making your experience smoother and more enjoyable. If you think you may be interested in participating in a clinical trial but don’t know where to start, you can try out Power, where you can find out if there are any trials you are eligible for and may benefit from in only a few minutes.