Top 20 Biopharma in Patient Engagement on Power
These 20 biopharmas have the most engaged patients on Power
Power has become the go-to destination for patients seeking clinical trials, so we know what motivates patients better than anyone. We did a quick analysis to figure out which companies were best at engaging patients in the discovery and sign-up process in 2023, and why.
Power’s top 20 list ranks mid-to-large-size biopharma sponsors by patient engagement with their trials on Power during clinical trial discovery and sign-up.

- Merck Sharp & Dohme LLC
- Cerevel Therapeutics, LLC
- Biohaven Pharmaceuticals, Inc.
- Chugai Pharmaceutical
- Otsuka Pharmaceutical
- Novo Nordisk A/S
- Oneness Biotech Co., Ltd.
- PTC Therapeutics
- Ionis Pharmaceuticals, Inc.
- H. Lundbeck A/S
- Neurocrine Biosciences
- Eli Lilly and Company
- Sumitomo Pharma America
- Boehringer Ingelheim
- Jazz Pharmaceuticals
- ModernaTX, Inc.
- Agenus Inc.
- Biogen
- Sun Pharmaceutical
- AbbVie
What are these sponsors doing so well?
People are most engaged with patient-centric trials.
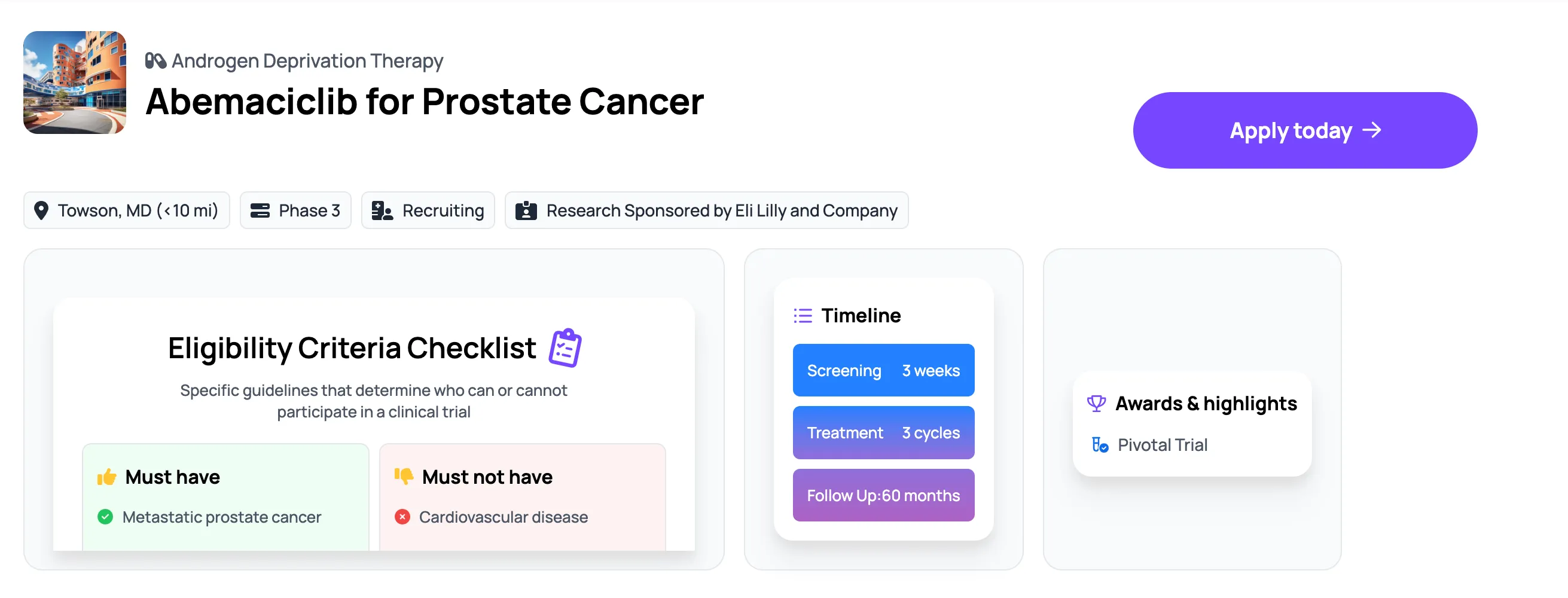
A prostate cancer trial with patient-centric design and pre-approved treatments has been trending on Power this year. Of all oncology trials, Eli Lilly’s phase III trial testing Abemaciclib has the most engaged patients.
The trial adds a new medication to two standard-of-care treatments instead of requiring a reset of the treatment regimen. It is also attractive to patients because it comes as a pill instead of a needle.
Abemaciclib has also already been FDA-approved for several types of breast cancers, so it has an understood safety profile.
Other top players are the phase I Boehringer Ingelheim trial for BI 456906, and the phase III Novo Nordisk A/S trial for Semaglutide. These two companies understood the reality of co-occurring diseases with obesity and designed trials specifically for those patients.
Both drugs are needles for weight loss. Boehringer Ingelheim is testing weight loss and liver problems, while Novo Nordisk is testing weight loss and Type II Diabetes. One of the factors contributing to successfully engaging patients is that they are targeting two conditions at once.
Patients are highly engaged with trials that study comorbidities because people rarely have a single condition. Since these trials emphasize and aim to understand these co-occurring conditions, they are better at attracting real, engaged patients.
Study page on Power for Eli Lily’s phase III trial testing Abemaciclib:
Patients are also highly engaged with trials that are making waves in the news and have responsive communications teams.
Another Eli Lily trial takes the top spot of all neurology trials on Power. Their phase III trial for Donanemab in Alzheimer’s Disease has made major waves as a breakthrough medication - one of the first significant breakthroughs in years. Since there is currently no cure for the disease, patients are highly motivated to try this promising new treatment.
And of all psychiatry trials on Power, Jazz Pharmaceuticals’ phase II trial for JZP150, a pill for PTSD, had the most engaged patients. It is in part doing well because they have a highly engaged research team - patients who find their trial via Power know that, if they qualify, they’ll be able to quickly book a screening visit.
Here’s how we analyzed the data.
First, we grabbed proprietary engagement data from our Patient Interest Graph on every trial in the country. Then, we tallied engagement from January to July 2023. We then shortened the boundaries of the data: only trials that were recruiting this year and run by companies with seven or more active trials qualified.
Next, we tallied engagement for every trial run by every sponsor on our shortlist. We divided total engagement by the number of trials run by each company - so companies with hundreds of trials wouldn’t outcompete smaller companies.
Understanding previous success is crucial to designing more engaging trials.
Biopharmaceutical sponsors that want to create a seamless recruitment process need creative ways to find the most motivated patients. First, they need strong data on what features make a trial most attractive to patients. Then, they need to design their trial to engage patients from the start.
Overall, the most successful biopharma companies have trials that are making waves in the news, have fast, patient-focussed communications teams, are pre-approved for other conditions, and study real patients with real co-occurring conditions.
We’ll plan to publish a new list in 2024. Any predictions for the top contenders?