Learn More About Psoriasis Research Studies
What Are Psoriasis Clinical Trials?
Psoriasis is a chronic skin disorder that causes skin cells to multiply at an accelerated rate. It can cause a rash with itchy, scaly patches, most commonly on the elbows, knees, lower back, and knees. However, the patches can appear anywhere on the body. There is no cure for psoriasis, and most treatment plans focus on managing its symptoms. Failing to treat psoriasis can lead to severe pain that affects the quality of life in patients.
Psoriasis usually develops in early adulthood but may occur at any age. Mild psoriasis only affects a few areas of the body, while severe cases could affect large portions of the body. These patches often heal on their own, only to return at a later point in the patient’s life. The symptoms of psoriasis often vary depending on the type. Common symptoms include plaques of red skin, disorders of the fingernail, and plaques of scales on the scalp.
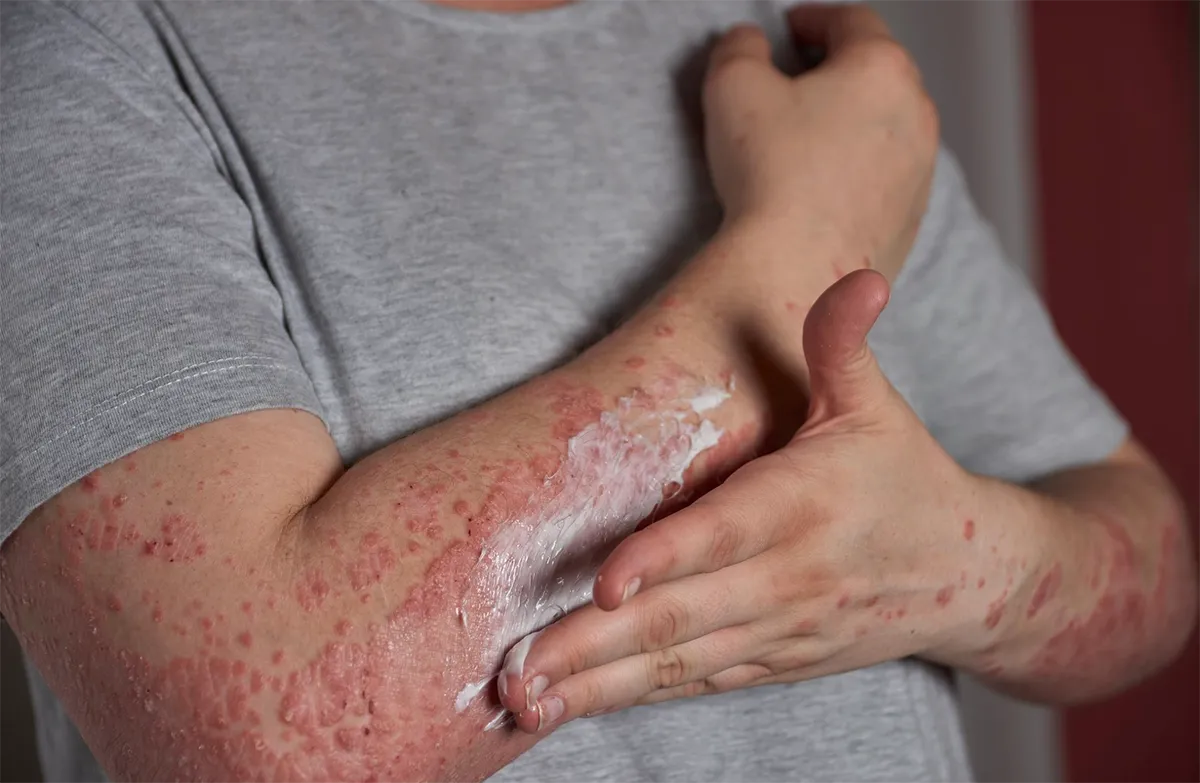
Several types of psoriasis include pustular psoriasis, guttate psoriasis, inverse psoriasis, and erythrodermic psoriasis, among others. Researchers don’t know what causes psoriasis, but common risk factors may include a genetic predisposition to the disease, infections, cuts, burns, and using certain medications. Doctors may prescribe treatments to manage the symptoms. Patients can also try to make changes to their lifestyle and develop coping strategies to improve their quality of life with psoriasis.
Psoriasis clinical trials are needed to understand its primary causes and improve diagnostic tools and treatment plans.
Why Is Psoriasis Being Studied Through Clinical Trials?
Psoriasis research studies currently focus on developing probiotics to see if they can provide an effective treatment. They are also investigating the use of ustekinumab (Stelara) in treating psoriasis. The drug blocks interleukin 12 and interleukin 23, which may result in better outcomes for patients. Gene-based gels have also been studied to see their efficacy on psoriasis.
One gel, AST-005, is based on a new technology called spherical nucleic acid that uses genetic material to block TNF-alpha (which triggers psoriasis). More clinical trials with a large sample size are needed to see if it can open up treatment for psoriasis.
Researchers have also investigated natural treatment with turmeric because it has been shown to block TNF-alpha. Turmeric is readily available as food, a supplement, and as a gel treatment.
Furthermore, it is well known that patients with psoriasis often struggle with getting high-quality REM sleep, leading to more skin problems. Research is currently exploring whether low-quality sleep can trigger certain skin diseases, including psoriasis.
What Are The Types of Treatments Available For Psoriasis?
There is no cure for psoriasis, and treatment plans aim to manage the symptoms associated with psoriasis. The exact treatment plan depends on the type of psoriasis and where it affects the body. Health practitioners will examine the patient’s skin, nails, and scalp to rule out other disorders and prescribe a treatment plan.
Most treatments are designed to stop skin cells from growing at an accelerated pace and removing scale. Common treatments include topical therapy (with ointments and cream), phototherapy (using light), oral medications, and injections. The go-to drug for treating mild cases of psoriasis is corticosteroids. They can be applied as oils, creams, ointments, foams, and gels. Hydrocortisone may be prescribed for sensitive areas of the body, such as the face and skin folds.
Skin cell growth can be slowed with the help of vitamin D analogs such as calcitriol and calcipotriene. These drugs may be used on their own or with topical corticosteroids. Of these, calcitriol causes less irritation of the sensitive areas but costs more than most topical corticosteroids.
Doctors may prescribe calcineurin inhibitors to reduce the buildup of scale. Pimecrolimus and tacrolimus have shown to be effective in treating thin areas of the skin, such as the eyes and face, where steroid creams could be harmful.
Light therapy, including sunlight, UVB broadband, and narrowband, excimer laser, and PUVA, are commonly used to treat severe forms of psoriasis. OF these, PUVA is the most severe and may have long-term side effects such as dry and wrinkled skin, a high risk of skin cancer, and higher sensitivity to the sun.
More studies are needed to develop novel methods for treating Psoriasis and managing its symptoms.
What Are Some Recent Breakthrough Clinical Trials For Psoriasis?
Psoriasis research studies aim to discover its underlying pathogenic mechanisms to pave the way for more effective treatment plans. Here are a few major breakthroughs in managing and treating psoriasis.
2019: Safety of Oral Administration of Probiotic Strains in Patients with Psoriasis - This randomized trial aimed to study the efficacy of a probiotic mixture in reducing the severity of psoriasis. Ninety participants (18-70 years old) were randomized into groups that received probiotics and placebo. The endpoint was the Psoriasis Area and Severity Index (PASI). It was found that 66.7% of participants who received probiotics and 41.9% of patients who received placebo demonstrated a 75% reduction in PASI. Furthermore, participants who took the probiotic mixture had a lower risk of relapse.
2019: Oxymatrine Therapy in Patients with Severe Plaque Psoriasis - This clinical trial assessed the effect of oxymatrine treatment on apoptosis and cell proliferation in skin lesions caused by psoriasis. Patients were treated with either oxymatrine or acitretin. Researchers performed correlations of the Psoriasis Area and Severity Index (PASI) and the proliferation and apoptosis index.
Oxymatrine demonstrated reduced PASI scores, which means that psoriasis lesions were significantly reduced. The mitotic index in the oxymatrine group also decreased after treatment. Although oxymatrine reduced proliferation, it inhibited apoptosis of cells in the skin lesion. It was concluded that oxymatrine is effective in treating severe plaque psoriasis and its efficacy was comparable with acitretin. Since acitretin may cause metabolic abnormalities, oxymatrine treatment may be a viable alternative for treating psoriasis.
2019: IL-17A Inhibition by Secukinumab for Treating Psoriasis - This clinical trial investigates the efficacy of Secukinumab, a human mAb, on treating psoriasis. Secukinumab inhibits Il-17A, which is central to psoriasis pathogenesis. Secukinumab may improve the manifestations of psoriasis with fewer side effects; however, in human subjects is lacking.
The study found that early reduction of IL-17A is ideal for plaque resolution.
2020: Treating Moderate to Severe Psoriasis Using Risankizumab - This clinical trial compared patient-reported outcomes (PROs) across three randomized groups: risankizumab vs. ustekinumab and placebo. The trials were conducted in 139 sites across North America, Europe, Japan, and the Asia Pacific region in patients with moderate to severe psoriasis. 997 patients were randomly assigned to 150 mg of risankizumab, 45 mg or 90 mg of ustekinumab, and a placebo.
Researchers used the Psoriasis Symptoms Scale (PSS), Dermatology Life Quality Index (DLQI), and Hospital Anxiety and Depression Scale (HADS) to compare the outcomes. A high number of patients with risankizumab achieved improved symptoms of moderate to severe psoriasis, including reduced psychological distress and improved HEQL, compared to the group that received ustekinumab or placebo.
2021: Efficacy of Guselkumab for Treating Psoriasis - This randomized controlled trial assessed the efficacy of guselkumab in treating psoriasis. 1829 patients were randomized to receive 100 mg guselkumab every nine weeks, placebo, or adalimumab. It was found that guselkumab maintained a high level of clinical response and improved patient outcomes in patients with moderate to severe psoriasis.
2021: Apremilast in Patients with Mild to Moderate Plaque Psoriasis - This study investigated the safety and efficacy of apremilast in patients with psoriasis. 595 participants were randomized to receive 30 mg apremilast twice every day and placebo. Many participants who received apremilast achieved Scalp Physician Global Assessment compared to the placebo group. In addition, there was significantly greater DLQI improvement compared to the placebo group. Side effects of apremilast included nausea, headache, vomiting, and diarrhea.
2021: Bimekizumab for Treating Moderate to Severe Plaque Psoriasis - Bimekizumab is an antibody that selectively inhibits both IL-17A and IL-17F. This randomized controlled trial aimed to find out the ideal dosing intervals for bimekizumab during maintenance therapy to maintain clear skin. 49 patients with psoriasis received 320 mg bimekizumab at weeks 0/4, 320 mg bimekizumab at week 16, and placebo. Researchers assessed efficacy, pharmacokinetics, immunogenicity, biopsy transcriptomic analyses, and safety.
The study found that the inhibition of IL-17A and IL-17F resulted in significant clinical responses and profound normalization of keratinocyte biology. Thus, bimekizumab maintenance dosing at intervals of 8 and 4 weeks is ideal.
2022: Secukinumab Dosing in Patients with Psoriasis Weighing 90 kg or More - This randomized controlled investigated the efficacy and tolerability of 300 mg Secukinumab every two weeks (Q2W) with 300 mg Secukinumab every four weeks (Q4W) in patients weighing 90 kg or more. It was found that the Q2W group that received 300 mg Secukinumab had superior results compared to the group with Q4W.
Who Are Some Of The Key Opinion Researchers and Institutions Conducting Psoriasis Clinical Trial Research?
The Psoriasis Research and Treatment Center at the Institute of Medical Immunology in Berlin focuses on chronic inflammatory skin diseases, including psoriasis, Pityriasis rubra pilaris, and Hidradenitis suppurativa. They are investigating the pathogenesis of psoriasis to develop novel medications and therapy. The center has researchers who have a thorough clinical and scientific background. They have collaborated with various institutions, including Sanofi–Aventis Deutschland GmbH and Bayer Schering Pharma AG, Berlin.
Center for Excellence for Psoriasis and Psoriatic Arthritis (CEPPA) at Oregon Health & Science University is dedicated to treating psoriasis and psoriatic arthritis. They provide individualized care to patients using a multidisciplinary and coordinated approach to manage the symptoms of psoriasis. A team of specialists from various backgrounds, including dermatologists, rheumatologists, and endocrinologists, work together to administer care. The institution is leading the research in psoriasis (including clinical trials).
James G. Krueger, M.D., Ph.D., leads the Laboratory for Investigative Dermatology at Rockefeller University in NYC. Krueger received his M.D. from Cornell University Medical College, where he also interned in internal medicine. He serves as a physician at the Center for Clinical and Translational Science at the Rockefeller University Hospital. Krueger led several clinical trials to study T cell, dendritic cell, and keratinocyte activation responses using techniques such as cell culture and biochemical analysis.